Abstract
The new WHO 2016 classification of lymphoid neoplasms requires the identification of Activated B-cell like (ABC) and Germinal center B-cell like (GCB) Diffuse large B-cell lymphomas (DLBCL) NOS as oncogenetic pathways and probably sensitivity to specific therapies differ between these two subtypes. Whereas RNA based signatures from FFPE DLBCL seem promising in reproducibility, still 15% to 20% of DLBCL NOS are of the Unclassified subtype and there is variability between gene expression profiling (GEP) and RNA signatures considering this unclassified subtype. Therefore, the immunohistochemical tool, used during the diagnostic process of the lymphoma, might be useful to maintain and develop in addition to the molecular tools. Hans and coworkers previously developed an immunohistochemical algorithm with 86% concordance with GEP among patients treated with Rituximab with antracyclin based regimens. However, some antibodies such as BCL6 are difficult to evaluate and might hamper reproducibility of scoring. We propose an alternative to the Hans algorithm using 4 easy to use antibodies while keeping the classification process fairly simple.
Methods
One thousand eight hundred forty-three patients with previously untreated de novo CD20+ DLBCL were enrolled in the GELA (Groupe d'Etudes des Lymphomes de l'Adulte)/LYSA (Lymphoma Study Association) LNH01-5B and LNH03-B clinical trials. A Tissue microarray of 854 tumors were built and immunostaining with CD10, MUM1, FOXP1 (Barrans' threshold, 0 vs Variable/Strong), BCL6, IGM, MYC, BCL2 was performed. 761 cases of DLBCL NOS had immunostains data available. For 150 of these patients, frozen tissue was available and GEP has been analyzed using GeneChip Human Genome HGU133 Plus 2.0 arrays (Affymetrix, Santa Clara, CA, USA). A new immunohistochemical algorithm was then developped and validated in concordance with GEP using a training set and a validation set (100 cases for training, 50 cases for validation). The first step was to choose the best and most parsimonious machine learning algorithm capable of predicting DLBCL subtypes. Then, logical rules were created based on the predicted probabilities of class membership using a probability threshold that optimize concordance between machine learning and logical rules predictions. xgBoost algorithm (boosted trees models) with 4 covariables was chosen, using a cut-off of 0.39 for predicted class membership probabilities.
Results
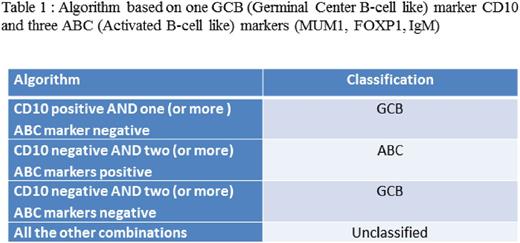
A new algorithm (Table 1) using CD10, MUM1, FOXP1 and IGM was then derived with an accuracy of 93% (IC95 [0.80 ; 0.98]) on the validation set, compared to GEP classification. This algorithm has four arms taking into account the GCB marker CD10 and independently one of the three ABC (MUM1, FOXP1, IGM) markers to classify as GCB, ABC or non classified. Hans algorithm had an accuracy of 0.88 (IC95 [0.75 ; 0.96]) on this same dataset. On the training and validation set combined, our algorithm re-classified correctly 7 GCB subtypes compared to the Hans algorithm.
Survival analysis on the remaining cases (611 patients) found an ABC vs GC hazard ratio (HR) of 2.0 (IC95 [1.47 ; 2.73]) for progression free survival (PFS) and 1.95 (IC95 [1.36-2.80]) for overall survival (OS), using ABC vs GCB classification of our new algorithm. In our series, Hans' algorithm found an ABC vs GCB HR of 1,74 IC95 [1,27-2,39] for PFS and 1,87 IC95 [1,29-2,71] for OS.
Conclusion
Our new algorithm shows excellent performance compared to the Gold Standard GEP classification, using only 4 immunostains, and allows for more accurate risk stratification both for PFS and OS than Hans'algorithm. Therefore, it can be used as a useful tool in addition to molecular signatures.
Bruneau: Innate Pharma: Research Funding. Delarue: Gilead: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Haioun: PFIZER: Consultancy, Honoraria; GILEAD: Consultancy, Honoraria; JANSSEN: Consultancy, Honoraria; Sandoz: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Casasnovas: BMS: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding. Morschhauser: Roche: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Gilead: Consultancy; Servier: Consultancy. Emile: Merck Serono: Honoraria. Tilly: Gilead: Honoraria; Immunogen: Honoraria; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria. Salles: Celgene: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Kite: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Servier: Consultancy, Honoraria; BMS: Consultancy; morphosys: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding. Jardin: Roche: Honoraria; celgen: Honoraria; Janssen: Honoraria. Molina: Merck Serono: Honoraria; Novartis: Honoraria; Takeda: Other: travel support to the ASH meeting.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal